Through ELSA we’ve been able to slow down the process and prepare – we know what is coming, but we're not scared.
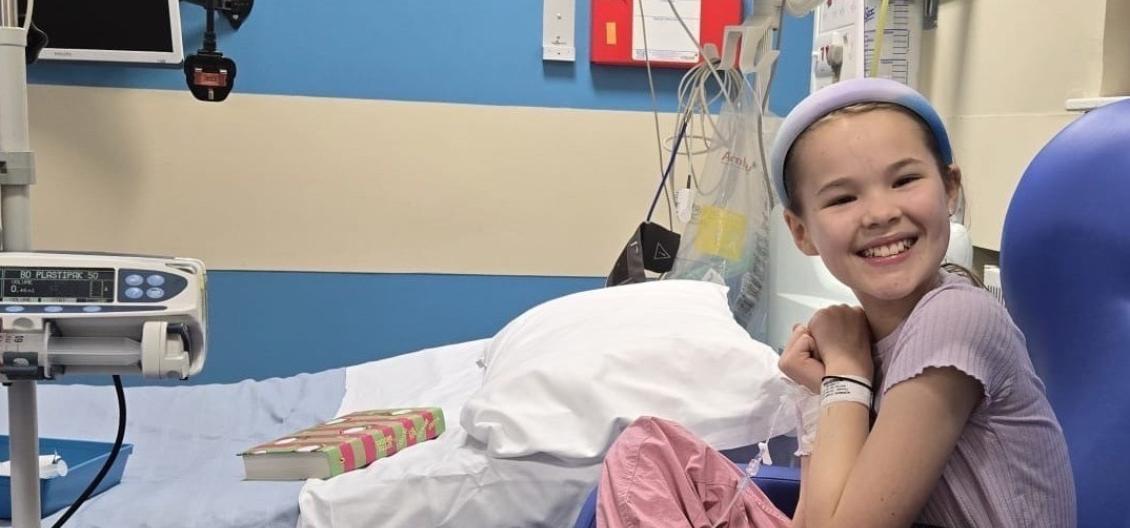
Amy is a mental health practitioner from the West Midlands who lives with type 1 diabetes. Her 12-year-old daughter Imogen took part in the ELSA study, which picked up she was in the early stages of type 1 diabetes.
As a result, Imogen became the second child in the UK to access a breakthrough new immunotherapy drug – teplizumab. Teplizumab hopes to slow the progression of type 1 diabetes and give her extra years without insulin therapy.
Imogen was able to access teplizumab through a managed access programme run by the drug company that allowed small numbers of people, who fit certain criteria, to try promising new treatments early.


